Two Dallas heart doctors wanted to help fight COVID-19. They might end up changing science.
By Nancy Brown, American Heart Association CEO

As cardiologists, Drs. James de Lemos and Sandeep Das are committed to their patients.
As professors and mentors, de Lemos and Das are devoted to their students.
As editors of a top scientific journal, they're gatekeepers for their peers.
As researchers, they seek answers for the greater good.
And as COVID-19 erupted into the biggest public health challenge of their lifetimes, they were …
"Powerless," de Lemos said.
"Sidelined," Das said.
In late March, about a week into the pandemic's reign, neither was on the front lines – or anywhere close. Others at UT Southwestern Medical Center in Dallas hated feeling out of the loop, too. So de Lemos and Das came up with a meaningful way to get folks involved.
They launched a project to compile all sorts of data about their hospital's COVID-19 patients. Think of it as a massive database that could identify everything from trends (Who's getting sickest? Who's recovering fastest?) to best practices (What helps? What doesn't?). In the science world, this database is called a registry.
Creating a registry to tackle a problem is common in science. But de Lemos and Das – who were plugged into the research world through their roles as journal editors – weren't aware of any reliable COVID-19 registries.
As UTSW started building such a registry, Das invited a few trusted colleagues around the country to join the project. Then de Lemos brought it to the American Heart Association, which offered spots to every hospital in the United States. Hospitals around the world heard about it and wanted in, too.
Told like that, it all makes sense – a good idea gaining momentum. Except, science rarely works that way. Or that quickly. And never the way this project is being run.
So why did it happen this time? Amid the frantic start of a pandemic that thinned resources and patience, why did so many people in so many places volunteer right away to take part in what was essentially an experiment?
The answer is profound, and profoundly simple.
This project tapped into the very thing that drove everyone involved into medicine: The desire to save lives. Only, this was far more than an aspiration to help mankind.
"Everybody working on this recognized the importance for their kids, their mother and father, their friends," de Lemos said. "Frankly, it's scary. Action is often the best antidote to fear, right?"
Time will tell what role this registry might have in mapping the best way to treat COVID-19 and/or create a vaccine. Time also will tell whether this project unlocks a new way of doing things that is sustainable under normal conditions.
Regardless, everything about this project will yield a fascinating case study. Consider this the first draft.
LAYING THE GROUNDWORK: 'I FEEL LIKE A FRAUD'
The virus that started in China and tore through Italy next tormented Spain. Yet, in early March, a safe harbor there was Alicante, a city along the Mediterranean Sea where de Lemos' daughter was spending her junior year of college.
De Lemos, his wife and their two sons planned a visit for spring break. After doing his due diligence, de Lemos felt comfortable making the trip.

They wound up there the same week the coronavirus tightened its grip on the United States. When President Trump demanded all Americans abroad return immediately, the de Lemos family snagged seats on the last flight out.
Back home in Dallas after three hectic days, de Lemos faced 14 days of quarantine before he could return to UTSW.
"I felt guilty," he said. "I've been at my institution for a long time and have a variety of roles. And here I was, not able to go to the hospital just as a pandemic was going on."
***
In early March, Das noticed his wife staying awake later than usual.
Most nights, she put their 9-year-old son to bed, then called her closest friends, the mothers of other third-graders who'd just put their kids to sleep, too. The calls usually ended by 10 p.m. As fears about the coronavirus heightened, she sometimes was on the phone until 1 a.m.
Das got the gist of those conversations the next morning.

In only a few days, she went from asking how the school could protect the kids to whether people should buy pulse-oximeter machines and oxygen canisters. A friend with an at-risk husband was so worried about a potential shortage of ventilators that she wanted to buy one.
"It was absurd, but it reflected the extent of their fear," Das said. "These were very well-informed people scrambling to figure out what they could do."
Meanwhile, as COVID-19 patients flooded into UTSW and its sister hospital, Parkland, the public hospital for Dallas County, Das began questioning what he was doing.
Seeing the tireless efforts of his colleagues, Das thought: "There are people out there on the front lines exposing themselves and I'm sitting at a computer working in my office most days. I feel like a fraud."
***
'HEROIC,' BUT NOT VERY HELPFUL
When de Lemos decided it was safe to visit Spain, and when Das answered his wife's questions, they did so not only as people who keep up with the latest trustworthy scientific information. They did so as people who help decide what is the latest trustworthy scientific information.
Doctors turn to scientific journals for such news. Among the go-to journals for cardiologists is Circulation, published by my organization, the American Heart Association. Since 2016, de Lemos has been executive editor, Das an associate editor.
In those roles they'd read reports about COVID-19. While de Lemos considered it "heroic" that doctors even found time to write papers, their scope was too narrow and their methods too varied to draw meaningful conclusions. Science needed reports based on standardized criteria from an enormous variety of hospitals.
Upon returning from Spain, de Lemos read more jarring, insufficient papers. But he soon set that aside.
He had something else to deal with at his main job.
***
UTSW is a teaching hospital, and de Lemos spent many years running the training program for cardiac "fellows," doctors on the final step of clinical and research training before becoming fully fledged cardiologists.
Although de Lemos is no longer in charge, he still trains fellows. Like him, they weren't allowed at the hospital. Also like him, they realized this could be the biggest public health challenge of their careers and wanted a role.
To brainstorm what they could do, de Lemos scheduled a Zoom meeting. He named it, "Fighting Back Against COVID."
During that conversation, the registry idea clicked.
De Lemos realized these eager young investigators could create the standardized criteria. They also could collect the data from UTSW, Parkland and other affiliated facilities.
But it had been years since de Lemos ran a registry. He needed a partner with more expertise. So he turned to a former fellow: Das.
***
TURNING PATIENT RECORDS INTO A VACCINE
How can scientists use data to conquer a virus?
To best explain, let's use a more relatable problem. Such as city planners trying to reduce traffic accidents.
The first step is gathering data. That starts with asking questions: Where are the most wrecks? The most deaths? What times do they occur? What are the traffic patterns?
Step two is turning all the information into a list of hot spots. Investigators would then find comparable intersections that aren't as dangerous and try figuring out why. Researchers also would round up changes that worked in other cities.
Once they settle on their most plausible solution, they reach step three, which is testing it. Say they decide to build a roundabout. After a certain interval (a year, maybe), they could compare the number of accidents and deaths from the roundabout to another, similar intersection that wasn't changed.
Translating this example to science, step one is building a registry, step two is using the registry and step three is running a randomized clinical trial, the gold standard of research. Any medicine approved to treat COVID-19, or vaccinate against it, will have to pass a randomized clinical trial.
So building a registry won't solve a problem. But it can guide the way.
***
Continuing the traffic example, let's say the roundabout worked so well that the city installed them at many dangerous intersections.
Once the pavement is dry, the planners would hand off responsibility to other overseers.
In business, that's quality control. In health care, it's called quality improvement, or QI.
The AHA has a nationwide council devoted to this. Das is among the leaders. He's also part of a smaller consortium devoted to QI for cardiovascular care. Those are the colleagues he first asked to join the COVID-19 registry.
Meanwhile, de Lemos bounced the idea off an executive vice president at UTSW, Dr. John Warner. In addition to Warner being a friend, he was two years removed from being president of the American Heart Association.
De Lemos asked him: Might the AHA want to take the lead on this?
***
WARP SPEED AHEAD
Warner knew the AHA was looking for a potentially game-changing way to take a big swing at COVID-19. He thought this could be it. He urged de Lemos to contact current AHA President Dr. Bob Harrington and me.
The email arrived at 10:28 a.m. on Sunday, March 29. The specificity matters because it proved to be like a starter's pistol for a scientific 100-meter dash.
In a race that would shatter many speed records, the first was this: Hospitals were invited to sign up for the AHA's COVID-19 Cardiovascular Disease Registry five days later.
***
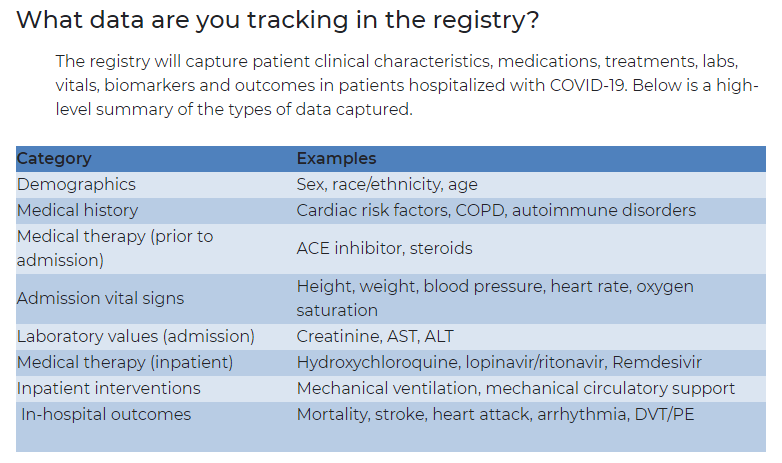
The first building block of the registry itself is the master questionnaire used to capture the data about every patient. It's called the Case Record Form.
The day after the Zoom call that started this whole process, de Lemos asked the fellows to start creating the form. He based the assignment on a simple premise: What would you want to know about COVID-19 patients from a cardiovascular standpoint?
Once the AHA got involved, staffers and volunteers who often work on registries tweaked it. This included more stroke-based questions under the guidance of Dr. Mitch Elkind of Columbia University, a member of the steering committee and the incoming president of the AHA. (He replaced Harrington on July 1.)
The seven-page form includes basic demographics, medical history and details specific to the patient's COVID-19 symptoms and treatment. Data is stripped of identifying information.
Normally, going from the idea for a registry to hospitals uploading CRFs takes at least a year, often two.
This was done in 24 days.
"It required 100% commitment from so many people," de Lemos said. "It was around-the-clock and weekends, and nobody blinked."
"They are doing this," Das said, "because it's the right thing to do – because getting information on how best to battle this pandemic is the right thing for America and for the world. It feels a little grandiose to say something like that, but it's true."
***
As of July 6, the registry included 34,634 lab reports and 9,232 patient records.
They came from 83 sites across 27 states. Another 63 sites in 18 more states are enrolled but have yet to upload data; more still are in the pipeline.
There's also been interest from hospitals in Argentina, Brazil, China, Columbia, India, Indonesia, Israel, Malaysia and Vietnam.
Everything about this exceeded expectations.
And this was only step one.
***
SEEKING THE TRUTH
Step two is sifting through the thousands of terabytes of data in hopes of connecting some dots.
Usually, researchers wanting to dig into the data must submit a proposal explaining what they want and why. A committee approves the projects deemed the most promising. Then a central analyzing center cranks out the requested information, one request at a time.
With this registry, any site that submits data can request data. They still need a committee's approval, but they won't have to wait for the information.
Data scientists at the researchers' institutions will get the data they need from the AHA's cloud-based Precision Medicine Platform. Hospitals can ask AHA data scientists for help with the analysis or even to do it for them.
The important innovation is that dozens of teams across the county will pursue different questions from a single set of data at the same time.
"We call it 'burst science,'" de Lemos said. "We think we'll be able to do many more analyses and research projects in a much shorter period of time."
Study proposals began arriving in late May. Approvals began rolling out June 26.
***

Now the big question: What will researchers find in this registry?
Some will look at the impact on people of different races and ethnicities. Others will compare patients in rural versus urban areas. There will be deep dives into whether certain treatments helped, hurt or did nothing.
"Once we start defining best practices, then we'll give feedback to hospital and health system administrators who can then use that information to change how they deliver care," Das said.
That feedback will come in the form of published papers. That's usually years into a registry; this time, it'll be months.
If all goes well, the registry could yield an AHA scientific statement. This doesn't carry the same weight as a guideline (the best treatment practices for doctors), but it's the next best thing.
Better still, one of these projects could lead to step three: a randomized clinical trial.
"The people designing the vaccine are working in a lab right now," Das said. "We hope to be sort of their Best Supporting Actor. We want to facilitate their efforts."
***
With COVID-19 cases in the U.S. topping 2.9 million and around 130,000 deaths, answers can't come fast enough. Plenty of other registries are being built in the global pursuit of answers.
While de Lemos would love for this registry to produce a breakthrough, he'd also love if it doesn't.
"If we put in all this work and things die down because someone else found the answer first, that would be a blessing," he said.
A blessing for everyone, including his family. Because one of his kids has an autoimmune condition.
***
No matter what happens, this project already has shown new ways of doing things.
And that's what science is all about.
Researchers often describe their work as pursuing the truth. Many also call it a team sport. This registry has revealed the best of both notions.
"It sort of restores your faith in academic medicine in general for this spirit of volunteerism and self-sacrifice and going above and beyond," de Lemos said. "I hate to use the word 'rewarding' in the setting of a pandemic, but this has been one of the most invigorating intellectual experiences of my research career."
A version of this story appeared on Thrive Global.
If you have questions or comments about this story, please email [email protected].







